Information
Particulate matter
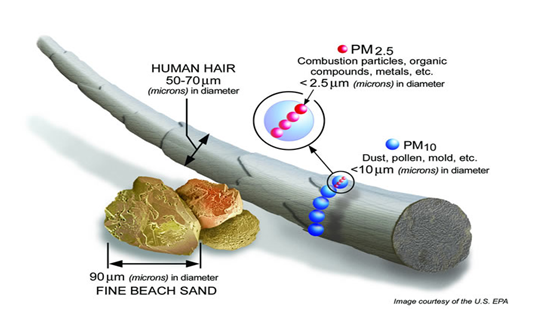
Particulate matter denotes all small solid and liquid particles that float around in the atmosphere. They can reside there for hours to months depending on their properties and on meteorological conditions. A particle floating around in a gas is called an aerosol. The behaviour of particles in an aerosol is determined by the properties of the particles (dimensions, form, density) and those of the gas (velocity, turbulence, composition). The term "aerodynamic diameter" has been developed to describe the behaviour of particles. This behaviour is determined by the dimensions, form and density of the particles. The aerodynamic diameter is defined as the diameter of a spherical particle whose behaviour in ambient air is identical to that of the particle under consideration, hereby assuming that the spherical particle has the same density as water. The PM10 particle fraction has an aerodynamic diameter less than 10 micrometer (μm), PM2.5 has a diameter of less than 2.5 μm. Particles with a diameter less than 0.1 µm are called ultrafine particles.
Sources
The particles can end up in the atmosphere through a natural cause (natural aerosol) or through human activities (anthropogenic aerosol). In both cases, they can be categorised as primary and secondary particles in terms of the way in which they are formed. Primary particles are emitted directly into the atmosphere or formed by mechanical fragmentation of coarser material (e.g. heavy metals in metal processing). The main emissions caused by humans originate from transport, industry, agriculture and building heating. Major natural sources of primary particulate matter are sea salt aerosol and wind-blown soil dust. Secondary particles are formed in the atmosphere by oxidation and transformation from gaseous components such as NH3, SO2, NOx or from organic compounds such as volatile organic compounds (VOCs).
Composition
The composition of secondary particles is highly complex. They are formed from the gaseous phase, and by condensation, where substances with the lowest vapour pressure condense faster than those with a higher vapour pressure. Fine particles can therefore exhibit a complex, layered structure. This is amplified by the fact that the available surface of all particulate matter in the atmosphere is delivered mainly by the small particles. Substances that are emitted in gaseous form (including dioxins) will therefore be deposited almost exclusively on the small particles. Heavy metals from foundries and traffic, PAHs, dioxin and soot are therefore present in the fine fraction.
Effects
Epidemiological studies show that the most severe health effects caused by air pollution are due to particulate matter, and to a lesser extent, to ozone. Inhalation of particulate matter causes irritation or damage to the pulmonary tissue. Particulate matter can cause both short and long-term health effects. According to the World Health Organisation (WHO), there is no safe threshold value below which no harmful effects occur. While short-term exposure to particulate matter complicates existing health problems such as respiratory infections and asthma, the health effects of long-term or chronic exposure are significantly more severe. Chronic exposure increases the risk of cardiovascular and pulmonary diseases and of lung cancer. Exposure to the current PM2.5 concentrations is estimated to reduce average life expectancy of the Belgian population by approximately nine to ten months (Amann et al., 2005). In the Flemish Region, particulate matter accounts for approximately three-quarters of lost years of healthy life due to environmental factors (MIRA, 2012). The PM2.5 fraction was found to have the strongest link with health effects, but effects were also demonstrated for the finer UFP fraction (particulate matter with diameter less than 0.1 μm) and the coarser 2.5-10 μm fraction (Brunekreef et al, 2005). Particulate matter includes Black Carbon (BC or diesel soot) and other combustion-related material, which in itself is not the most toxic component of the smaller PM particles, but it is a carrier of various chemical, toxic substances.
In addition, particulate matter has adverse effects on climate change and ecosystems. It contributes to the degradation of treated surfaces which therefore need to be cleaned more often (the so-called soiling effect) and has, depending on the composition, a corrosive effect on material and cultural heritage. Particulate matter has both a cooling (sulphate aerosols) and warming (black carbon) effect, and therefore also plays a role in climate change.
EU and WHO standards
PM10
| standard | protection objective | averaging period | value |
max number of exceedances | date by which value is to be met |
|---|---|---|---|---|---|
| EU limit value | human health | 1 day | 50 µg/m³ | 35 | 1 january 2005 |
| EU limit value | human health | 1 year | 40 µg/m³ | 1 january 2005 | |
| WHO guideline | human health | 1 day | 45 µg/m³ | 3 | |
| WHO guideline | human health | 1 year | 15 µg/m³ |
PM2.5
| standard | protection objective | averaging period | value |
max number of exceedances | date by which value is to be met |
|---|---|---|---|---|---|
| EU target | human health | 1 year | 25 µg/m³ | 1 january 2010 | |
| EU limit value | human health | 1 year | 25 µg/m³ | 1 january 2015 | |
| EU limit value | human health | 1 year | 20 µg/m³ | 1 january 2020 | |
| WHO guideline | human health | 1 day | 15 µg/m³ | 3 | |
| WHO guideline | human health | 1 year | 5 µg/m³ |
